BASAL GANLGIA-THALAMOCORTICAL DYNAMICS IN SPEECH
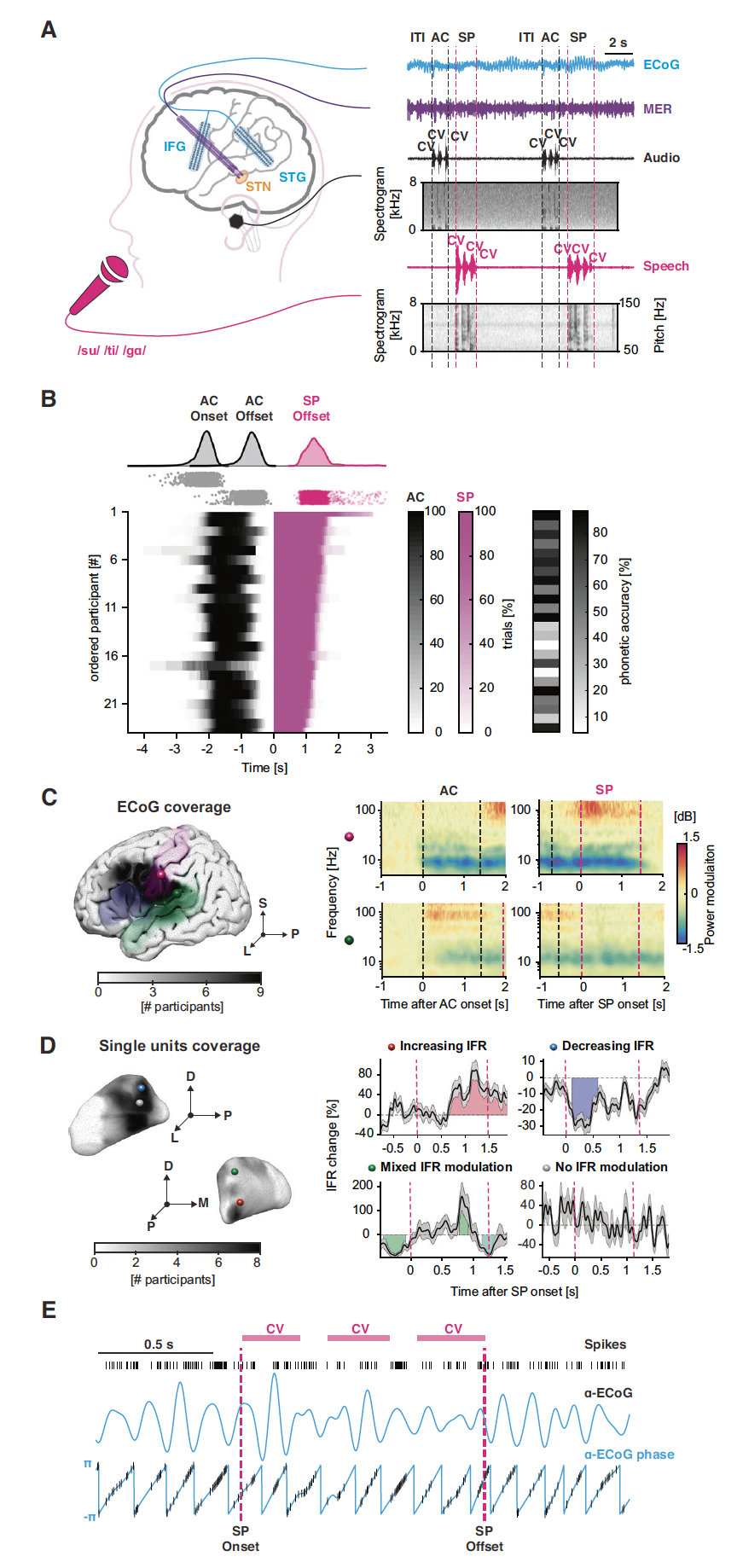
The Brain Modulation Lab has pioneered the study of basal ganglia and thalamic contributions to human speech production, an area that had been largely overlooked by prominent speech neuroscience models. This work was funded through two BRAIN Initiative U01 awards. By leveraging the rare opportunity to record simultaneously from the cortex and subcortical nuclei in awake humans undergoing deep brain stimulation surgery, our group was the first to establish that subthalamic nucleus (STN) neurons encode aspects of speech production, revealing temporally distinct populations that reflect both early planning and late motor execution phases of speaking (Lipski et al., Journal of Neuroscience, 2018). We subsequently demonstrated that STN single-neuron spike timing is phase-locked to theta-alpha oscillations in posterior perisylvian cortex, and that the timing of this coupling predicts speech sound accuracy (Vissani et al., Nature Communications, 2025). We also showed that STN neurons are dynamically phase-synchronized to cortical oscillations during movement, independent of firing rate changes (Lipski et al., Journal of Neurophysiology, 2017), and that the STN influences sensory and motor cortex during force production (Alhourani et al., Cerebral Cortex, 2020). Our work has further demonstrated that auditory cortex directly influences the STN during speech motor planning in a manner modulated by lexicality (Weiss et al., Neurobiology of Language, 2023), that articulatory gain is represented across the cortico-basal ganglia network (Dastolfo-Hromack et al., Cerebral Cortex, 2022), that the STN and cortex show distinct lexicality effects during reading aloud (Chrabaszcz et al., Journal of Neurolinguistics, 2021), that the thalamus actively participates in lexical processing during reading aloud in a lateralized and region-specific manner (Wang et al., Journal of Neuroscience, 2022), and that fundamental differences in aperiodic neural dynamics distinguish cortical from subcortical activity (Bush et al., Cerebral Cortex, 2024).
A landmark contribution of our lab has been the discovery of a putative hyperdirect pathway from auditory cortex to the STN in humans — a connection not observed in non-human animal models that may have co-evolved with the capacity for speech (Jorge et al., Cell Reports, 2022). By mapping antidromic evoked potentials from STN stimulation across the opercular cortex, we showed that the entire frontal-parietal-temporal speech network, including sensory areas, sends monosynaptic projections to the STN in a topographically organized fashion. Collectively, our findings reveal that the basal ganglia are deeply integrated into the cortical networks supporting speech planning, production, and perception, and that this integration operates through oscillatory coupling mechanisms that cannot be captured by firing-rate models alone. These discoveries underscore the need for mesoscale neuromodulation technologies capable of interfacing with fine-grained neural populations within subcortical nuclei, in order to both advance our understanding of these circuits and develop more precise, next-generation therapeutic interventions for neurological disorders that affect speech and movement.
Because the insights described above depend on invasive intracranial recordings in human research participants who do not directly benefit therapeutically from the research components of their surgery, our lab is deeply committed to demonstrating the safety and clinical integrity of these procedures. We co-led a multi-center retrospective study across four BRAIN Initiative–funded sites, encompassing 367 DBS surgeries, which confirmed that intraoperative research electrocorticography does not increase complication rates beyond those expected for standard DBS surgery, with no cortical hemorrhages observed and an overall complication rate of 1.91% (Sisterson et al., Neurosurgery, 2021). We further demonstrated that temporary placement of subdural ECoG strip electrodes for research does not compromise the accuracy of DBS lead implantation, with neither radial nor Euclidean targeting error differing significantly between research participants and non-participants (Kons et al., Operative Neurosurgery, 2022). Together, these studies provide the evidence base that enables the ethical advancement of invasive human neuroscience and ensure that the pursuit of fundamental discoveries about brain circuit function does not come at the cost of clinical care.
The Brain Modulation Lab's body of work constitutes a paradigm challenge for the field of speech neuroscience. Dominant models of speech motor control are overwhelmingly corticocentric, treating the basal ganglia — when they consider them at all — as a monolithic gate that either permits or suppresses motor programs. Our data argue for something far more nuanced: the STN is not merely gating speech but is actively participating in the sensorimotor computations that produce it, at a temporal resolution and functional specificity that no current model accommodates. The discovery of a putative auditory-to-STN hyperdirect pathway — absent in non-human animals — positions the STN as a hub that integrates sensory feedback with motor plans in real time, potentially supporting the rapid auditory-motor coordinate transformations that fluent speech requires. The spike-phase coupling work extends this by showing that when STN-cortical interaction is delayed, speech errors occur, providing a mechanistic account of how basal ganglia dysfunction may lead to speech deficits in Parkinson's disease and potentially other disorders. Our finding that cortical and subcortical local field potentials have fundamentally different aperiodic spectral properties further implies that analytical methods developed for cortical signals cannot simply be applied to subcortical recordings — an important caveat for the growing field of closed-loop neuromodulation. Ultimately, our scientific findings write the requirements specification for next-generation devices: they need to record and stimulate at the spatial scale of the neural populations that matter, and our safety work provides the ethical foundation on which that translational effort can proceed.