RESPONSIVE NEUROSTIMULATION NEUROPHYSIOLOGY IN EPILEPSY
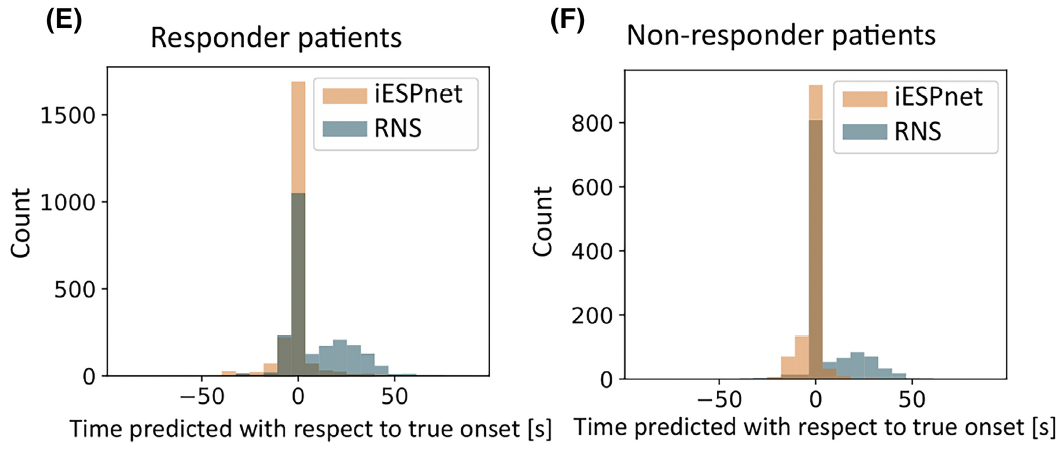
In parallel with expanding clinical indications, we have made foundational contributions to understanding the mechanisms and optimizing the technology of closed-loop brain stimulation. In a landmark study, we discovered that the therapeutic mechanism of RNS is not primarily acute seizure termination (which accounted for only approximately 5% of stimulated seizures), but rather progressive, indirect modulation of seizure network properties, including spontaneous ictal inhibition, frequency modulation, and network fragmentation, that emerged over weeks to months and predicted clinical response (Kokkinos et al., JAMA Neurology, 2019). To manage and analyze the vast quantities of intracranial EEG data generated by the RNS device, we developed BRAINStim (Biophysically Rational Analysis of Individual Neural Stimulation), a custom software platform that extracts, organizes, and enables spectral analysis of chronic electrocorticographic recordings (Sisterson et al., Neuroinformatics, 2020). We further quantified a specific frequency modulation response biomarker, an indirect electrophysiological effect of stimulation characterized by emergent shifts in the spectral content of seizure patterns, and demonstrated its potential for automated detection and use in optimizing stimulation parameters (Venkatesh et al., Journal of Neural Engineering, 2021). Building on these analytic foundations, we developed deep learning systems for expert-level automated detection of intracranial EEG seizure patterns in RNS recordings (Constantino et al., Frontiers in Neurology, 2021), subsequently extending this work to create iESPnet, a neural network capable of both detecting the presence and predicting the onset time of electrographic seizure patterns across patients with diverse seizure presentations (Peterson et al., Epilepsia, 2023).
The finding that RNS appears to operate primarily through progressive network modulation rather than acute seizure termination has practical implications for how device programming and outcome evaluation are approached, suggesting that longitudinal electrophysiological changes may be more informative biomarkers than acute stimulation responses. We are now pursuing this translational opportunity through an NINDS-funded R61/R33 project to validate distinct categories of intracranial electrographic seizure pattern modulation (iESPM) as both prediction and response biomarkers in responsive neurostimulation, across two large academic RNS databases (MGH and Mount Sinai). Our goal is to enable objective, electrophysiology-based guidance that is currently unavailable in clinical practice. The broader shift from focus-guided to network-guided epilepsy surgery, to which our clinical data and conceptual contributions have contributed, may also inform neuromodulation strategies for other circuit-based brain disorders. Given that epilepsy surgery remains substantially underutilized, continued efforts to build the evidence base for thalamic neuromodulation and to develop scalable analytic infrastructure are important priorities for the field.